Subprojects
Mathematical model on generation of ectopic tertiary lymphoid follicle in context of renal transplantation
After renal transplantation there is a risk of induction of a host immune response against the graft, which may lead to rejection and loss of function of the transplanted organ. Currently available prognostic factors are not always sufficient to predict a developing rejection early enough to adapt the immunosuppressive therapy in time.
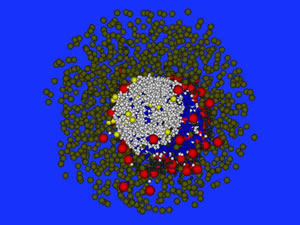
Three-dimensional simulation of the formation of lymphoid B cell follicles using the Delaunay-Object-Dynamics methodology.
Image by Michael Meyer – Hermann, HZI Brunswick, Germany
In order to utilize the full predictive potential of routinely obtained tissue samples (so-called "protocol biopsies"), we investigate early indications of newly formed lymphatic structures. Potentially, these structures develop long time before detection of antibodies directed against the graft (the donor-specific antibodies). With the help of dynamic mathematical models we aim at determining early signs of the formation of such displaced (ectopic) lymphoid structures.
The generated knowledge on early signs of ectopic lymph structures and on their relevance for graft rejection are integrated in computer-assisted analysis workflows to evaluate microscopic images from clinically collected biopsies. We expect insights into the underlying immunological processes enabling innovative and individually tailored therapy approaches for patients in need.
CONTACT
Prof. Dr. Michael Meyer-Hermann
Helmholtz Zentrum für Infektionsforschung
» mmh@theoretical-biology.de
» www.systems-immunology.de
» www.helmholtz-hzi.de
Image and data mining of spatial immune cell patterns to develop novel prognostic tissue markers
The aim of Subproject 2 within the SYSIMIT consortium is to generate a prognostic model for acute rejection, with particular emphasis on "developing acute humoral rejection" and a risk-calculation model for hereditary breast cancer. These models will be based on correlating extensive quantitative image analyses of differently stained tissue sections (Fig. 1) with their associated clinical data. To achieve this, a Definiens AG software platform for image analysis, visualization and statistical data evaluation is used as a foundation; this platform will be extended with new prototype components within the scope of the project.
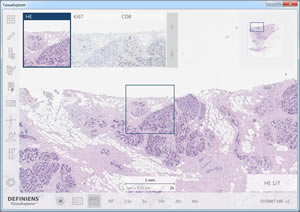
Definiens TissueExplorer™ software features an automatically aligned display of multiple sections of a tissue block, which enables the analysis of protein-co-expression and cell co-occurrence patterns. The image shows the H&E-stained section in the main window, used to visualize tissue morphology, and in the smaller windows the corresponding regions stained with Ki67 to highlight proliferating cells and CD8 to mark T-cells.
Image by Ralf Schönmeyer, Definiens AG, Munich, Germany
The task of the automated image analysis is to detect and measure relevant structures, such as tumor cells, T-cells and macrophages (Fig. 2), in (immuno-)histochemically stained whole-slide images. The analysis involves developing parameters to quantitatively describe spatial organization of objects such as tertiary lymphoid organs, representing a context which co-determines the functions of these objects.
The results of automated measurements are stored in a unified format to make them available to project partners. The development of these tissue-based diagnostic tests requires the latest bioinformatics methods. The most important challenge is to reduce the almost-infinite number of possible measurements to a smaller set of robust and meaningful descriptors; a process that involves high-performance, parallel computing.
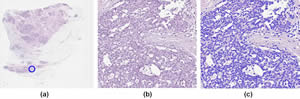
Identification of cell nuclei in an H&E-stained breast-tissue sample: (a) overview of the digitized tissue section. The location of the detailed view (b) is indicated by a blue circle. (c) automated detection of nuclei quantitatively characterized by shape, texture and spatial organization (context). These parameters are the basis to generate prognostic models.
Image by Ralf Schönmeyer, Definiens AG, Munich, Germany
Those descriptors will provide optimal correlation with the clinical data and deliver clinically decisive prognostic models. All developments are conducted in close collaboration with project partners, who bring clinical expertise and experience of model generation. Finally, the prognostic value of these new predictors will be evaluated in a (research-related) clinical routine environment.
CONTACT
Dr. Ralf Schönmeyer
Definiens AG, Research
» rschoenmeyer@definiens.com
» www.definiens.com
A model of T-cell – epithelial cell interaction in lymphocytic lobulitis at the interface of hereditary breast cancer and adjacent tissue
In this subproject, we will develop a dynamic mathematical model of the interaction between immune cells and epithelial cells in normal lobular epithelium, and in lobular epithelium adjacent to, or infiltrated by cancer cells. The rationale for this scientific objective is the "lymphocytic lobulitis" (LLO), a recurrent, currently poorly understood pattern of inflammation frequently observed in hereditary breast cancer.
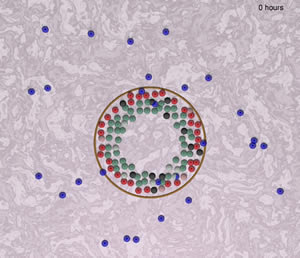
Framework of a model to simulate the interaction between "immune cells" (blue) and epithelial cells of an intralobular tubular gland (green, red, black, pink). The "immune cells" in this sreenshot are a default placeholder (not yet defined) moving according to literature-curated rules, interacting with epithelial cells that undergo a continuous cell turnover under cyclical hormonal changes.
Image by Juan Carlos Lopez and Haralampos Hatzikirou, Technical University Dresden, Germany
The distinctive pattern of inflammation could inform us about underlying inflammatory processes associated with development and spread of cancer, and has a potential for discovery of novel biomarkers.
Framework of a model to simulate the interaction between "immune cells" (blue) and epithelial cells of an intralobular tubular gland (green, red, black, pink). The "immune cells" in this sreenshot are a default placeholder (not yet defined) moving according to literature-curated rules, interacting with epithelial cells that undergo a continuous cell turnover under cyclical hormonal changes.
The observed interaction patterns between immune cell and epithelial cells at the invasive tumor edge will be compared with non-neoplastic epithelium in adjacent breast tissue, in larger distance to the tumor, and with normal epithelium. Joining spatial statistical analysis of multiple sections of lobules in different parts of the immunohistochemically stained tissue slides along with novel methodologies adapted from material sciences, we will draw conclusions about the interactions between immune cells and lobular epithelial cells. In combination with literature-curated rules, we will develop a mathematical model that will allow for the study of the functional role of LLO, for example its relation to tumor invasion. The model will be validated and iteratively improved by increasing numbers of observations on real cases. Advanced prototype versions of the mathematical model will then be used to study the effect of perturbations of the system, including simulations of the potential effect of novel therapies targeting the inflammatory tumor microenvironment (iTME). Finally, in close collaboration with subproject 4, we will attempt to conclude on the prognostic role of LLO to in hereditary breast cancer, and potentially propose additional criteria for biopsy evaluation that are currently not part of the routine diagnostic workflow.
CONTACT
Dr. Haralampos Hatzikirou
Center for Advancing Electronics Dresden - cfaed
» haralambos.hatzikirou@tu-dresden.de
» www.hatzikirou.net
» www.cfead.org
Prognostic value of the inflammatory response to hereditary breast cancer focusing on lymphocytic lobulitis
A common feature of hereditary breast cancer is a prominent inflammatory infiltrate that can be observed directly adjacent to the tumor, or in more distant parts of the normal mammary gland. This inflammation of normal mammary gland lobules, often referred to as lymphocytic lobulitis, is sometimes even detected before a malignant tumor is established, in prophylactically removed breast tissue of women with exceptionally high familial risk who decide to take this step in order to prevent cancer onset.
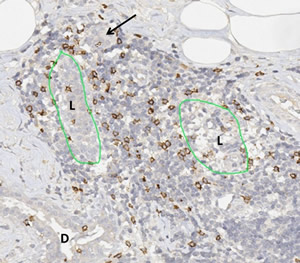
Lymphocytic lobulitis in a case of hereditary breast cancer. The massive lymphocytic infiltrates have destroyed the structure of the glandular lobulus and pushed aside the tubular components of the former lobule (L). The arrows shows a totally isolated tubular structure in the periphery of the inflammatory infiltrate. Adjacent to the former lobule a section of a ductal structure (D). The immunohistochemical staining for CD8 highlights a specific lymphocyte subpopulation, the cytotoxic T-lymphocytes, which have the capability to destroy other cells through direct contact.
Image by Friedrich Feuerhake, Hannover Medical School, Hannover, Germany
This project evaluates the prognostic value of inflammatory reactions to familial breast cancer, and aims at using this phenomenon as a diagnostic marker for risk estimation. We use a novel approach of knowledge-based image analysis, a method that has been recently established in geosciences to analyze satellite-based remote sensing image data of the earth surface adopted to the requirements of digital microscopic image analysis. Complex pattern recognition in digital histopathology images is joined with sytems biology-based evaluation of clinical, serological, and genomic data.
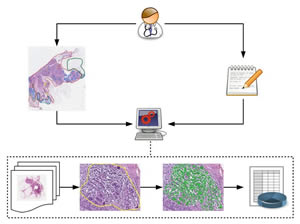
Overview depicting the methodical approach of knowledge-based image analysis, based on systematic annotation of training images, formalized expert knowledge, successive training of machine learning algorithms, and iterative improvement of the model. The results of this complex image analysis is integrated as "spatial dimension" in the systems medicine approach to data reduction and integrative analysis together with multimodal biomedical data of other sources.
Image by Gregory Apou, ICube Laboratory, University of Strasbourg, France
The approach is based on the analysis of fully digitalized histological sections, called whole slide images (WSI), a technology that opens novel perspectives but also brings up challenges regarding data and image management, and computational power for image processing. The novel analysis workflow will be tailored to the specific requirements of clinical applications in two retrospective, and one prospective cohort of familial breast cancer patients, always with focus on lymphocytic lobulitis. Particular attention is given to temporal and spatial aspects, since inflammatory responses are highly dynamic three-dimensional processes, of which a histological section can, by definition, only reflect a two-dimensional "snapshot". To this end, we collaborate closely with subproject 3, which establishes a spatially and temporally variable model of relevant aspects underlying lymphocytic lobulitis. We expect that the comprehensive analysis integrating the spatio-temporal dimension with conventional biomedical data analysis will lead to a better mechanistic understanding of lymphycytic lobulitis and inflammatory tumor infiltration, result in novel prognostic markers and may enable targeted immunomodulatory therapy approaches in familial breast cancer.
CONTACT
Prof. Dr. Friedrich Feuerhake
Institut für Pathologie, Medizinische Hochschule Hannover
» feuerhake.friedrich@mh-hannover.de
» www.mh-hannover.de/pathologie.html
